Lab Prof. Dr. H. Poeck
Targeted immunomodulation in cancer
Inspired by his clinical work focusing on myeloid neoplasms, allogeneic stem cell transplantation (allo-SCT) and cellular therapies with genetically modified T cells (CAR T cells), Prof. Poeck's research group is investigating anti-tumor responses, triggering of resistance mechanisms and tissue regeneration in the treatment of cancer patients with immunotherapies. In particular, we would like to determine the importance of (i) specific signaling pathways of the innate immune system and its modulation via genome modification, (ii) the different components of the stool and tissue microbiome including the role of nutrition / diet and (iii) tumor-derived factors (e.g. tumor-derived extracellular vesicles (EVs)) with regard to therapy success and development of resistance as well as for tissue regeneration in the context of the above-mentioned immunotherapies. His research thus combines the highly relevant areas of microbiome, nutrition, stem cell transplantation and cellular therapies including cell engineering and nucleic acid-based therapeutics.
We pursue both a “forward and reverse translation approach” in which interesting results from the mouse or from the patient (e.g. identification of certain microbial metabolites/signatures in CAR-T and allogeneic stem cell transplanted patients that are associated with an improved response) are translated into high-quality preclinical models (e.g. patient-derived organoid co-culture models, xenograft and immunocompetent mouse models) to further investigate the efficacy and molecular mechanisms of the new combinations in models with clinical relevance. The ultimate goal is then to translate this into an early clinical trial (Investigator Initiated Clinical Trials (IITs)).
With continued large inter-individual differences in therapy response, the aim of our research work is to use the above information for the development of new, targeted combination therapies to improve the response rates of these immunotherapies. At the same time, new biomarkers for predicting clinical outcomes will be identified and adverse immune-mediated side effects such as graft-versus-host disease (GvHD), cytokine release syndrome (CRS) or immune effector cell-associated neurotoxicity syndrome (ICANS) after CAR T therapy will be minimized. To achieve this goal, a number of local, national and international collaborations have been established.
Regular hashtags that describe the group environment:
International, friendly, open-minded, motivated, creative, diligent, hard-working, social
Scientific keywords that describe the group and its research:
Nucleic-Acid Therapeutics, microbiome, metabolites, nutrition, CAR T cells, allogeneic hematopoietic stem cell transplantation, cellular engineering
-
Postdoctoral Fellows:
(2020-present)- Markus Perl, MD, (since 2020, experiments running)
Research Focus: Cellular Engineering, CRSIPR/Cas9, CAR-T cells, Vectordesign, Microbiome, clinical translation - Kaiji Fan, PhD (since 2020, experiments running)
Research Focus: Immunomodulation via extracellular vesicles, Tissue Microbiota in GVHD and GvL, human organoid cultures - Paul Heinrich, Computational Biologist, PhD (since 2022, experiments running)
Research Focus: Transcriptomics, Metabolomics, single cell techniques - Simon Holzinger, Computational Biologist, PhD (since 2025, experiments running)
Research Focus: Epigenetics, microbiome analysis, single cell techniques - Severin Gütter, PhD (since 2024)
Research Focus: extracellular vesicles, CAR-T cells - Sascha Göttert, PhD (since 2024)
Research Focus: Metabolites as immunomodulators in allogeneic transplantation - Saba Sameri, PhD (since 2025)
Reseacrh
Graduate (PhD) students
(2020-present)- Sascha Göttert, PhD Student (since 2018, experiments finished)
- Chiara Suriano, PhD Student (started 2022, experiments running)
- Dhyani Shah, PhD Student (since 2022)
- Omer Khalid, PhD Student (started 05 / 2022, experiments running)
- Misbah Tariq, PhD Student (since 2024, experiments running)
- Maria Driesslein (PhD Student) (since 2024, experiments running)
- Suqi Li, PhD Student (since 2024, experiments running)
Medical Students (cand. med)
(2020-present)- Luisa Marie-Becker, cand. med. (since 2021, experiments finished)
- Leonard Knödler, cand. med. (since 2021, experiments running)
- Julian Scherer, cand. med. (since 2024, experiments running)
- Konstantin Herfeld, cand. med. (since 2023, experiments running)
- Sabrina Kunz, cand. med. (since 2020, experiments finished)
- Markus Perl, MD, (since 2020, experiments running)
-
2024 Translationsgruppe „TANGO“ (BZKF)
2023 European Research Council (ERC) Consolidator Grant 2023
2023 Basic Science Award (EBMT 2023)
2022 Basic Science Award (EBMT 2022)
2022 Excellence Funding Programme for Established Scientists by German Cancer Aid
2018 EMBO Young Investigator Programme
2017 Mechthild-Harf Research Grant by DKMS
2016 European Hematology Association (EHA) Research Grant
2013 Feodor Lynen Research Fellowship for Experienced Researchers (AVH foundation)
2010 Walter-Schulz-Forschungspreis
2009 Vincenz-Czerny-Forschungspreis (DGHO)
2006 Kulturpreis Bayern, gestiftet vom Bayerischen Staatsministerium für Wissenschaft,
Forschung und Kunst (beste Doktorarbeit der LMU)2004 Harvard-Stipendium der LMU-HMI-Allianz (PJ Tertial Innere Medizin)
2003 – '05 Stipendium der Bayer Science and Education Foundation
-
- DFG Collaborative Research Center (CRC) TRR221/A08
- DFG-Sachbeihilfe/ Einzelantrag (PO 1575/5-1)
- DFG Collaborative Research Center (CRC) 1371/P05
- Leibniz-Institute for Immunotherapy (LIT)
- Excellence funding program der Deutschen Krebshilfe (70114547)
- Bayerisches Zentrum für Krebsforschung (BZKF)
- European Research Council (ERC) (Consolidator grant)
-
- Heidegger S, Stritzke F, Dahl S, Daßler-Plenker J, Joachim L, Buschmann D, Winter C, Fan K, Enßle S,Li S, Perl M, Görgens A, Haas T, Thiele Orberg E, Göttert S, Wölfel C, Engleitner T, Rad R, Herr W, Giebel B, Ruland J, Bassermann F, Coch C, Hartmann G and Poeck H. Harnessing nucleic acid sensors in tumor cells to reprogram biogenesis and RNA cargo of extracellular vesicles for T-cell-mediated cancer immunotherapy. Cell Rep Med. 2023 Sep 19;4(9):101171. [IF: 11.7].
By “reprogramming” the immunomodulatory function of these tumor-derived EVs, we developed an innovative, personalized way to induce T cell-based antitumor immunity. Our results will provide the basis for the production of immunostimulatory EVs as a new, cell-free, multifunctional and personalized cancer therapy.
- Joachim L, Göttert S, Sax A, Steiger K, Neuhaus K, Heinrich P, Fan K, Thiele-Orberg E, Kleigrewe K, Ruland J, Bassermann F, Herr W, Posch C, Heidegger S, Poeck H. The microbial metabolite desaminotyrosine enhances T-cell priming and cancer immunotherapy with immune checkpoint inhibitors. eBioMedicine. Volume 97, 2023, 104834, ISSN 2352-3964, https://doi.org/10.1016/j.ebiom.2023.104834. [IF: 9.2].
Identification of a microbial metabolite (DAT) as a promising candidate for a new microbial-based therapeutic to improve the efficacy of cancer immunotherapies and especially in patients previously treated with broad-spectrum antibiotics (to overcome “dysbiosis”).
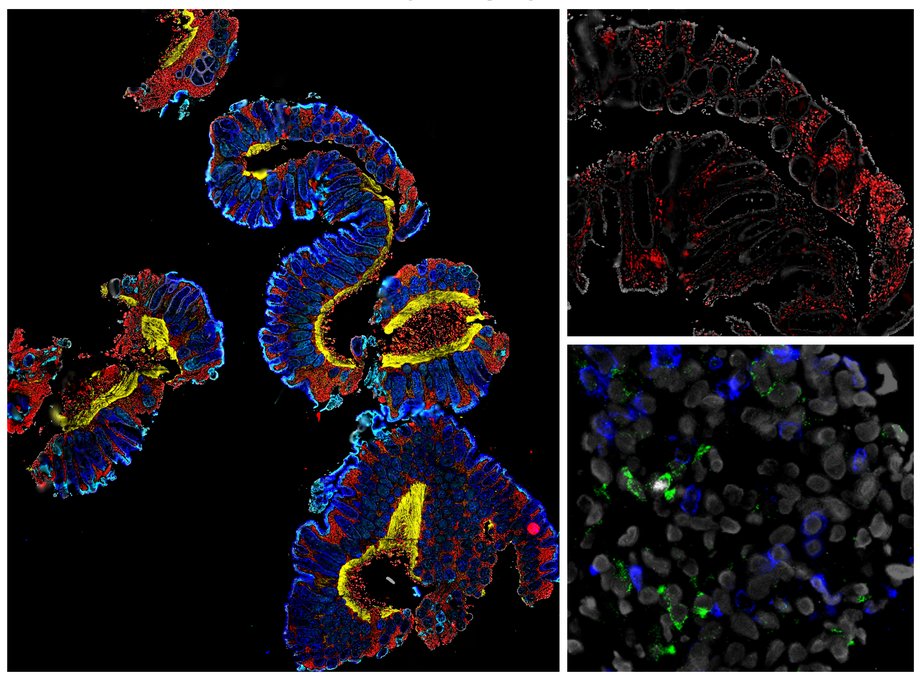
- Thiele Orberg E, Meedt E, Hiergeist A, Xue J,Heinrich P, Ru J, Ghimire S, Miltiadous O, Lindner S,Tiefgraber M, Göldel S, Eismann T, Schwarz A, Göttert S, Jarosch S, Steiger K, Schulz C, Gigl M,Fischer JC, Janssen KP, Quante M, Heidegger S, Herhaus P, Verbeek M, Ruland J, van den Brink MRM, Weber D, Edinger M, Wolff D, Busch DH, Kleigrewe K, Herr W, Bassermann F, Gessner A, Deng L, Holler E, Poeck H. Bacterial and Bacteriophage Consortia are Associated with Protective Intestinal Immuno-modulatory Metabolites in Allogeneic Stem Cell Transplantation Patients. Nat. Cancer 2024; Jan 3. doi: 10.1038/s43018-023-00669-x. [IF: 24.6].
Discovery of “microbial metabolites” and definition of a new risk index (“immuno-modulatory risk index”) for predicting clinical responses in allo-SCT patients. Foundation for clinical testing of a new “Precision FMT product” with defined microbiota-metabolite cocktails in allo-SCT patients to both improve response rates and reduce side effects (GvHD).
- Heidegger S*#, Wintges A *, Stritzke F, Bek S, Steiger K, Koenig PA, Göttert S, Engleitner T, Öllinger R, Nedelko T, Fischer JC, Makarov V, Winter C, Rad R, van den Brink MRM, Ruland J, Bassermann F, Chan TA, Haas T, Poeck H. RIG-I activation is critical for responsiveness to checkpoint blockade. Sci Immunol., 2019 Sep 13;4(39). [IF: 19.2].
First Identification of Intratumoral RIG-I gene expression as a biomarker to select patients that will likely benefit from anti-CTLA-4 therapy; clinical RIG-I targeting in patients may also increase overall response rates of checkpoint inhibitor-based immunotherapy.
- Heidegger S*#, Kreppel D*, Bscheider M, Stritzke F, Nedelko T, Wintges A, Bek S,Fischer JC, Graalman T, Kalinke U, Bassermann F, Haas T, Poeck H*#. RIG-I activating immunostimulatory RNA boosts the efficacy of anticancer vaccines and synergizes withcheckpoint blockade. eBioMedicine. 2019 Mar6. pii: S2352-3964(19)30135 [IF: 9.2]
We developed strategies to overcome aberrant tumor cell-intrinsic RIG-I signaling and associated cancer resistance to ICB immunotherapy.
- Fischer JC*, M Bscheider*, Gabriel Eisenkolb*, Lin CC, Wintges A, Otten V, Lindemans CA, Heidegger S, Rudelius M, Monette S, Porosnicu Rodriguez K, Calafiore M, Liebermann S, Liu C, Lienenklaus S, Weiss S, Kalinke U, Ruland J, Peschel C, Shono Y, Docampo M, Velardi E, Jenq R, Hanash A, Dudakov JA, Haas T, van den Brink MR *# and Poeck H*#. RIG-I/MAVS and STING signaling promote epithelial integrity during irradiation and immune-medited tissue injury; Sci Transl Med. 2017 Apr 19;9(386)[IF: 16.9].
As current immunosuppressive approaches are associated with increased risks of infections and relapse of malignancies, selective RIG-I or STING activation with defined ligands to improve GVL and reduce GvHD following allo-HSCT to treat leukemia and other blood disorders could have a major clinical impact.
- Jankovic D, Ganesan J, Bscheider M, Stickel N, Weber FC, Guarda G, Follo M, Pfeifer D, Tardivel A, Ludigs K, Bouazzaoui A, Kerl K, Fischer JC, Haas T, Schmitt-Gräff A, Manoharan A, Müller L, Finke J, Martin SF, Gorka O, Peschel C, Ruland J, Idzko M, Duyster J, Holler E, French LE, Poeck H*#, Contassot E*#, Zeiser R*#.The Nlrp3 inflammasome regulates acute graft-versus-host disease. J Exp Med. 2013 Sep 23;210(10):1899-910. [IF: 14.2].
Our study uncovered that selective targeting of NLRP3 may provide a novel therapy for the treatment of steroid-refractory GvHD.
- Poeck H*, Bscheider M*, Gross O*, Roth S, Finger K, Hannesschläger N, Schlee M, Rebsamen M, Rothenfusser S, Barchet W, Akira S, Inoue S, Endres S, Peschel C, Hartmann G*, Hornung V* and Ruland J*. Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1beta production. Nat Immunol. 2010 Jan;11(1):63-9. [IF: 28.4]
We show that upon recognition of RNA viruses and RIG-I ligands, RIG-I triggers a dual signaling cascade that is required for proinflammatory responses and inflammasome activation. Together with the paper below, this paper paved the way for the clinical development of RIG-I agonists against cancer therapy (phase I/II clinical trials).
- Poeck H*, Besch R*, Maihoefer C, Renn M, Tormo D, Morskaya S, Kirschnek S, Gaffal E, Landsberg J, Hellmuth J, Schmidt A, Anz D, Bscheider M, Schwerd T, Berking C, Bourquin C, Kalinke U, Kremmer E, Kato H, Akira S, Meyers R, Häcker G, Neuenhahn M, Busch D, Ruland J, Rothenfusser S, Prinz M, Hornung V, Endres S, Tüting T & Hartmann G. 5’-triphosphate siRNA: turning gene-silencing and RIG-I activation against melanoma. Nat Med. 2008 Nov;14 (11):1256-63 [IF: 59.2]
We identified RIG-I as a selective target for bifunctional 5´-triphosphate siRNAs (3p-siRNA) that combat tumors by combining gene-silencing, RIG-I activation and selective apoptosis induction in one siRNA-molecule.
- Gross O*, Poeck H*, Bscheider M, Dostert C, Hannesschläger N, Endres S, Hartmann G, Tardivel A, Schweighoffer E, Tybulewicz V, Mocsai A, Tschopp J, Ruland J. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009 May 21;459(7245):433-6. [IF: 54.4].
First description that the Nlrp3 inflammasome is responsible for sensing of fungi and initiation of host defense mechanisms.
- Heidegger S, Stritzke F, Dahl S, Daßler-Plenker J, Joachim L, Buschmann D, Winter C, Fan K, Enßle S,Li S, Perl M, Görgens A, Haas T, Thiele Orberg E, Göttert S, Wölfel C, Engleitner T, Rad R, Herr W, Giebel B, Ruland J, Bassermann F, Coch C, Hartmann G and Poeck H. Harnessing nucleic acid sensors in tumor cells to reprogram biogenesis and RNA cargo of extracellular vesicles for T-cell-mediated cancer immunotherapy. Cell Rep Med. 2023 Sep 19;4(9):101171. [IF: 11.7].